Start strong with ~3x longer PFS in 1L
LUTATHERA + SSA demonstrated statistically significant mPFS compared with SSA alone1,2
Median PFS (Primary End Point)
LUTATHERA + SSA: ~2 years PFS in 1L patients
PFS was defined as the time from randomization to first documented disease progression or death due to any cause. Centrally assessed according to RECIST v1.1 criteria1
The primary PFS analysis data cutoff was July 20, 2023. Median duration of follow-up: 23.2 months (from randomization to cutoff date)2
Follow-up for OS is ongoing
There is a planned final analysis of OS in the follow-up period after patients complete their treatment, retreatment, or crossover phase. Follow-up period was at least 6 months and up to 3 years, or until end of study, whichever comes first1
1st radioligand therapy with 1st-line evidence in newly diagnosed patients demonstrated in a phase 3 study1-3,*
NCCN Clinical Practice Guidelines In Oncology (NCCN Guidelines®) select recommendations5
PRRT with lutetium Lu 177 dotatate (LUTATHERA®) is NCCN Guidelines® recommended as a systemic therapy option for SSTR+, well-differentiated GEP-NET patients*
Grade 1/2
- NCCN Category 2A Preferred as an alternative first-line option for certain patients with locoregional advanced disease and/or distant metastases of gastrointestinal or pancreatic NETs (Ki-67 ≥10% and clinically significant tumor burden)†
Grade 3
- Category 2A for locally advanced and/or metastatic NETs with unresectable, clinically significant tumor burden or evidence of progression and favorable biology
- Category 2B for locally advanced and/or metastatic NETs with unresectable, asymptomatic, low tumor burden and favorable biology
NCCN Guidelines recommendations for 1L are based on data from the NETTER-2 study
†Consider for pancreatic NETs.
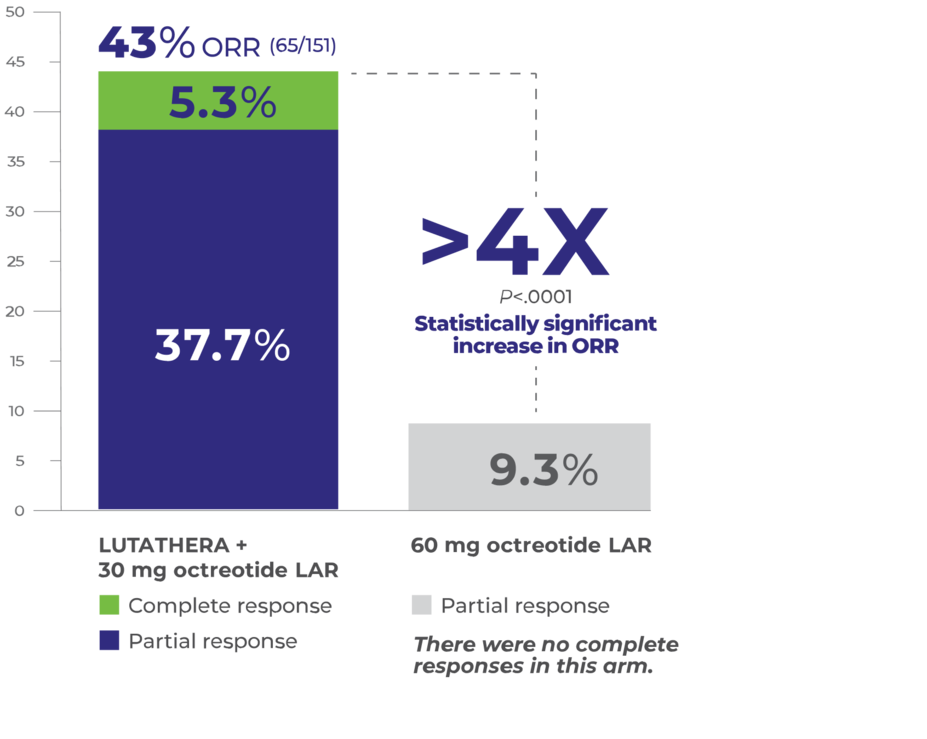
>4x more patients achieved a response with LUTATHERA4
Overall Response Rate (Full Analysis Set)4
Duration of Response2
Median DOR was 23.3 months with LUTATHERA + SSA (95% CI, 18.4-NE) vs NE* (95% CI, 2.3-NE) with SSA alone2
NETTER-2: Time to deterioration in patient-reported measures
Median TTD* in months (95% CI)2,6
HRQOL, functioning, and symptoms were assessed using EORTC QLQ-C30 and QLQ-G.I.NET211
98.7% of patients across arms completed their baseline EORTC QLQ-C30 questionnaires. The compliance rate was above 98% at the first post-baseline assessment and remained ~80% throughout the first year of treatment4
QOL was assessed on treatment only; as of the cutoff date, 80% of patients in the 60-mg octreotide LAR arm had discontinued treatment vs 48% of patients in the LUTATHERA arm4
†EORTC QLQ-C30 is an integrated questionnaire for assessing the HRQOL of cancer patients participating in clinical trials using a scale of 0 to 100, with high scores representing high or healthy levels of functioning/high QOL. The EORTC QLQ-G.I.NET21 was developed as an add-on module to the QLQ-C30 to better assess the symptoms and issues most relevant to patients with NETs.1,7
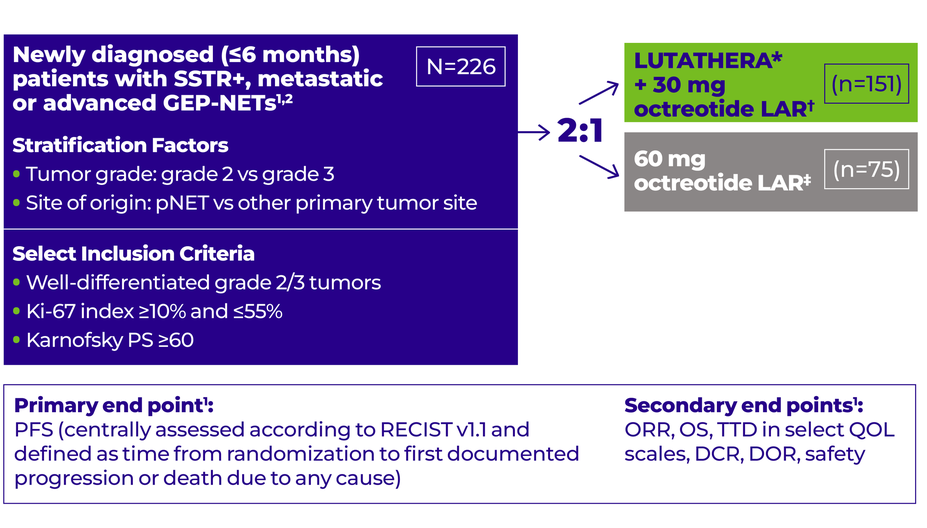
Study design
NETTER-2: A phase 3, randomized, open-label, multicenter study in 226 patients with metastatic or advanced GEP-NETs2
*LUTATHERA: 4 cycles, 7.4 GBq (200 mCi) every 8 weeks (+/-1 week). Investigators had the option of enrolling patients for retreatment with LUTATHERA if they had previously received and benefited from 4 doses as initial treatment.1,2
†Octreotide LAR: 30 mg every 8 weeks during LUTATHERA treatment and every 4 weeks after.1,2
‡Octreotide LAR: 60 mg every 4 weeks. Crossover from the 60-mg octreotide LAR arm to the LUTATHERA arm was allowed after patients experienced progression.1,2
LUTATHERA is the FIRST AND ONLY approved radioligand therapy with proven results in a clinical trial of >200 1L§ patients1-3
§In NETTER-2, 44 patients (19.5%) received prior treatment in the absence of progression, including CAPTEM (1 patient), everolimus (1 patient), and SSAs (42 patients, with the majority receiving 1 or 2 doses).2,4
NETTER-2 included patients with1:
Metastatic or locally advanced, inoperable GEP-NETs
A diagnosis within 6 months prior to the study
NETTER-2 excluded patients with1:
Documented RECIST progression to previous treatments for the current GEP-NET
Prior treatment with radioligand therapy
Prior external beam radiation therapy to >25% of the bone marrow
Any previous systemic therapy for GEP-NETs administered for >1 month or ≤12 weeks prior to randomization in the study
Any previous radioembolization, chemoembolization, and/or radiofrequency ablation for GEP-NETs
Surgery ≤12 weeks prior to randomization in the study
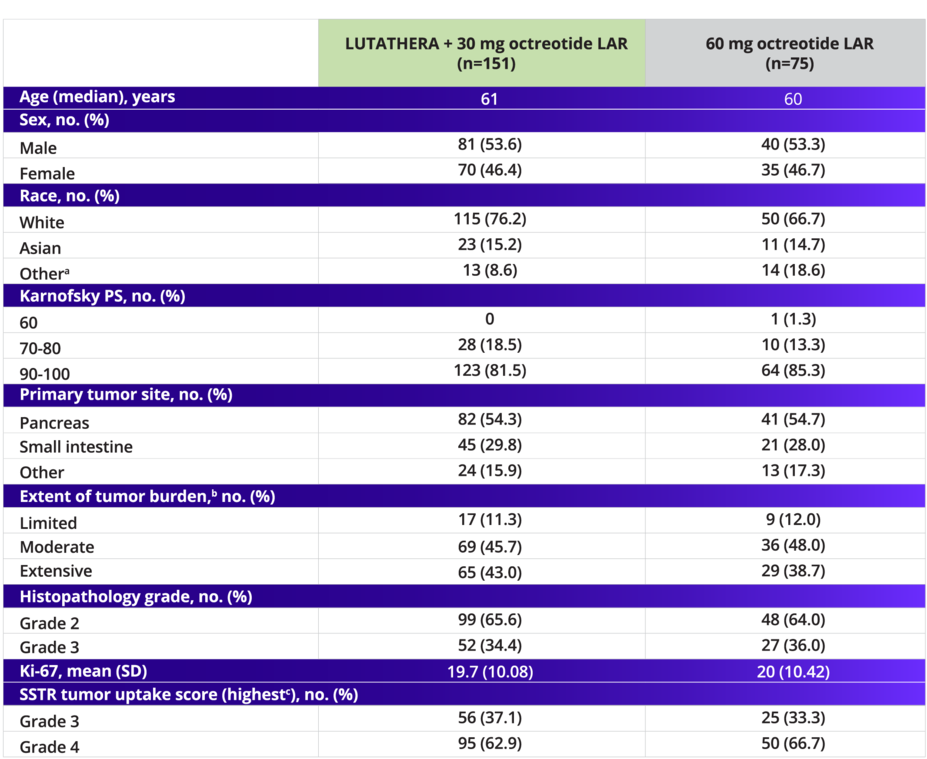
NETTER-2 demographics and disease characteristics were well balanced4
Baseline Demographics and Disease Characteristics
In the NETTER-2 trial, ~65% of patients had grade 2 NETs and ~35% had grade 3 NETs2
bExtent of tumor burden was based on central assessment.4
cHighest SSTR tumor uptake score is based on local assessment.4
1L, first line; CAPTEM, capecitabine and temozolomide; DCR, disease control rate; DOR, duration of response; EORTC, European Organisation for the Research and Treatment of Cancer; GBq, gigabecquerel; GEP-NETs, gastroenteropancreatic neuroendocrine tumors; GHS, global health status; HR, hazard ratio; HRQOL, health-related quality of life; LAR, long-acting release; mCi, millicurie; mPFS, median progression-free survival; NCCN, National Comprehensive Cancer Network; NE, not evaluable; NETs, neuroendocrine tumors; ORR, objective response rate; OS, overall survival; pNET, pancreatic neuroendocrine tumor; PFS, progression-free survival; PRRT, peptide receptor radionuclide therapy; PS, performance score; QLQ-C30, 30-item Quality of Life Questionnaire; QLQ-G.I.NET21, 21-item Gastrointestinal Neuroendocrine Tumor Quality of Life Questionnaire; QOL, quality of life; RECIST, Response Evaluation Criteria in Solid Tumors; SD, standard deviation; SSA, somatostatin analogue; SSTR, somatostatin receptor; SSTR+, somatostatin receptor-positive; TTD, time to deterioration.


![LUTATHERA + SSA prolonged PFS in patients with newly diagnosed, grade 2/3 GEP-NETs, with a 72% reduction in risk of progression or death (HR, 0.28 [95% CI, 0.182-0.418]; P<.001). The median PFS in the LUTATHERA arm was 22.8 months (95% CI, 19.4-NE) vs 8.5 months (95% CI, 7.7-13.8) in the control arm of 60 mg octreotide](https://usim.beprod.lutathera-hcp.com/sites/lutathera_hcp_com/files/styles/common_width_927/public/2025-07/9542-037_72km_chart_may_lighboxed_rgb_desktop.png?itok=_kESGihI)