Safety
NETTER-1 Safety Profile
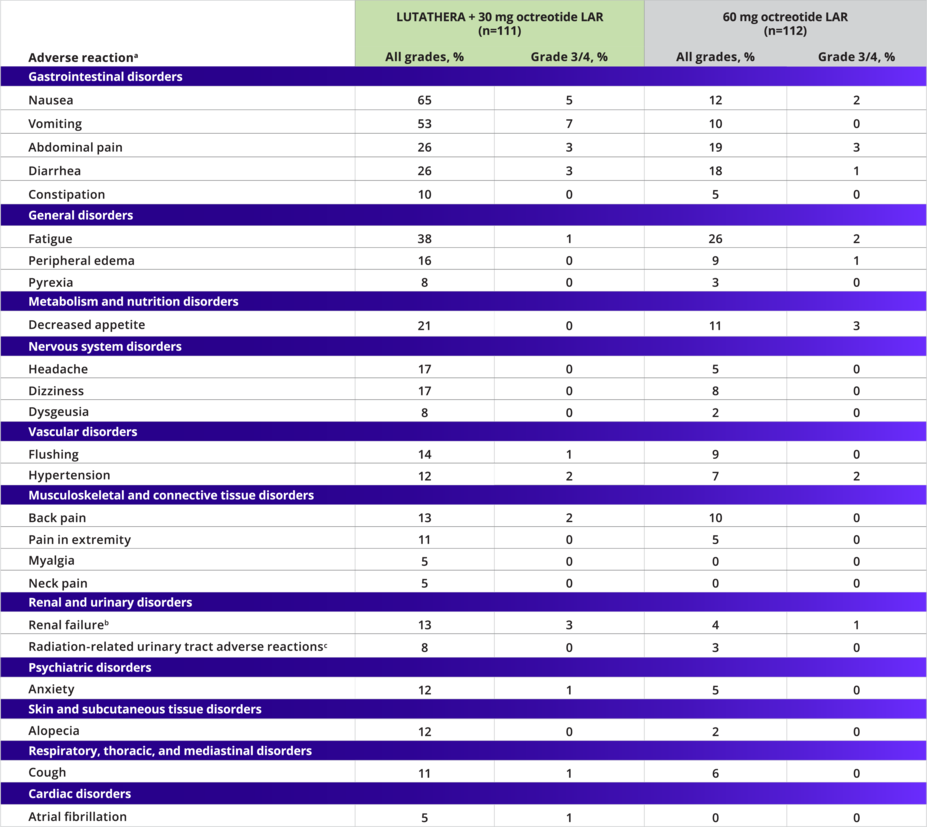
Adverse reactions1
Most of the adverse reactions seen in the LUTATHERA arm were grade 1/21
Adverse Reactions Occurring at a Higher Incidence in the LUTATHERA Arm
(Between-Arm Difference of ≥5% [All Grades] or ≥2% [Grade 3/4])1
bIncludes the terms glomerular filtration rate decreased, acute kidney injury, acute prerenal failure, azotemia, renal disorder, renal failure, and renal impairment.1
cIncludes the terms dysuria, micturition urgency, nocturia, pollakiuria, renal colic, renal pain, urinary tract pain, and urinary incontinence.1
The most common grade 3/4 adverse reactions with a higher incidence in the LUTATHERA arm were lymphopenia (44%), increased GGT (20%), vomiting (7%), nausea (5%), increased AST (5%), increased ALT (4%), hyperglycemia (4%), and hypokalemia (4%).1
ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; LAR, long-acting release.
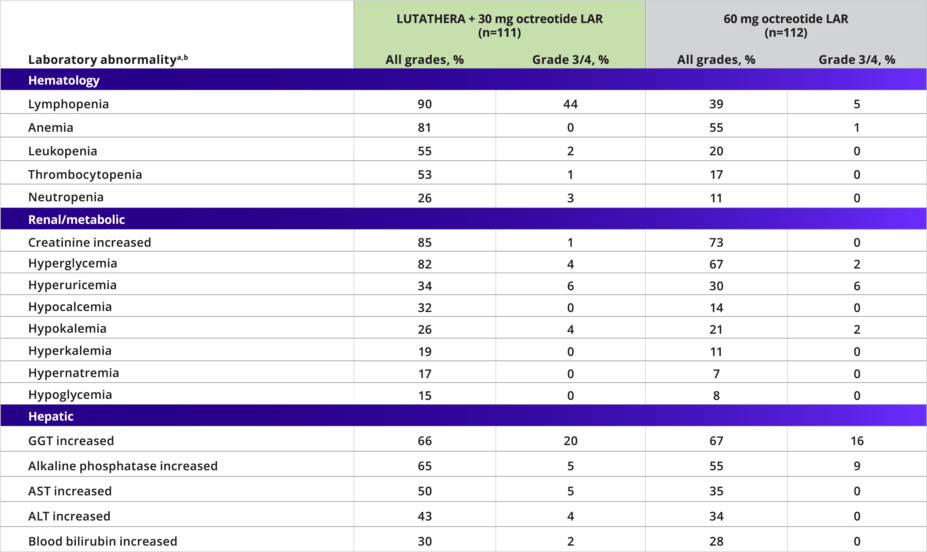
Laboratory abnormalities1
Established safety profile in the pivotal study for LUTATHERA1
Laboratory Abnormalities Occurring at a Higher Incidence in the LUTATHERA Arm (Between-Arm Difference of ≥5% [All Grades] or ≥2% [Grade 3/4])1
aValues are worse grade observed after randomization.1
bNational Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. Only displays laboratory abnormalities occurring at a higher incidence in patients treated with LUTATHERA (between-arm difference of ≥5% [all grades] or ≥2% [grade 3/4]).1
Dose reduction and discontinuation
6% (7 of 111) of patients required a dose reduction, and 13% (14 of 111) of patients discontinued LUTATHERA.1