About GEP-NET Patients
Identifying Patients for LUTATHERA
LUTATHERA can be used to treat SSTR+ GEP-NETs in the foregut, midgut, and hindgut1
Choose early LUTATHERA for your patients who are newly diagnosed or have progressed on an SSA1-3
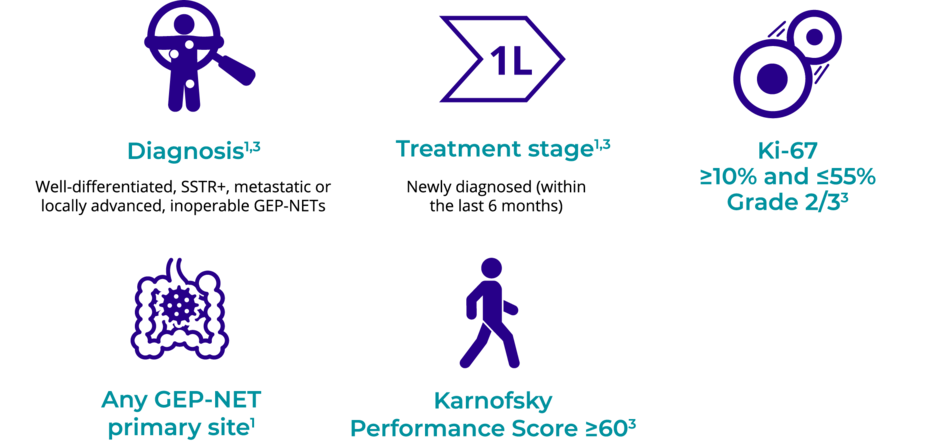
LUTATHERA is for patients with newly diagnosed GEP-NETs1,3
Consider these characteristics
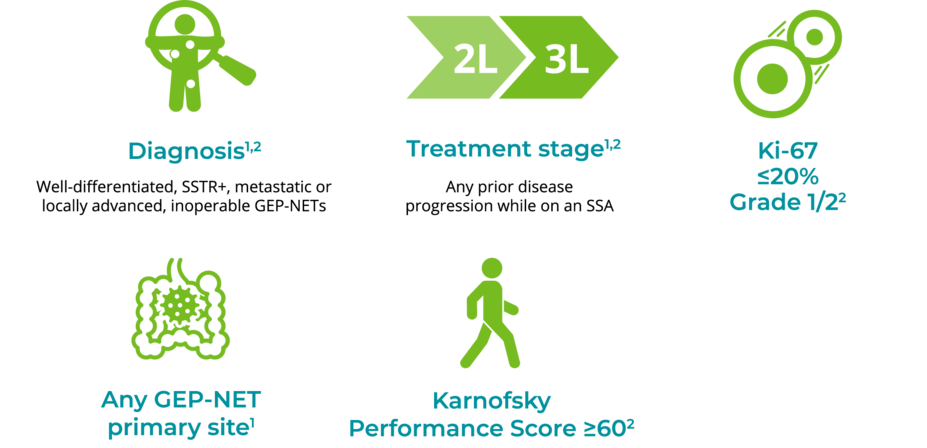
LUTATHERA is for patients with GEP-NETs after SSA progression1,2
Consider these characteristics
Which of your patients is ready to start strong with LUTATHERA?
1L, first line; 2L, second line; 3L, third line; GEP-NETs, gastroenteropancreatic neuroendocrine tumors; SSA, somatostatin analogue; SSTR+, somatostatin receptor-positive.